January 7, 2008 - Chinese-based Medical device maker Mindray Medical International Limited today announced updates on new product launches.
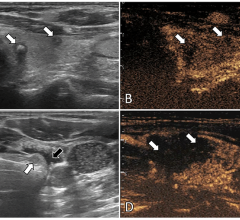
In December 2007 the company released the M5, a portable diagnostic ultrasound system for international markets. The M5 will target traditional markets such as radiology and OB/GYN as well as Point-of-Care-Testing centers such as emergency and operating rooms, intensive care units and general practice offices.
In 2007 the company released seven products including the Beneview T5 patient monitoring device, PM-60 pulse oximeter, BS-400 biochemistry analyzer, MR-96A enzyme-linked analyzer, MW-12A microplate washer, BS-120 biochemistry analyzer and the M5 portable diagnostic ultrasound system.
The PM-7000, a multi-parameter patient monitoring device, received FDA 510(K) clearance in the fourth quarter 2007.
The company plans to release its BC-5300 and BC-5380 compact five-part hematology analyzers, targeted for mid-to-high-end hospitals in China and medium-end labs in international markets. They are expected to receive CE Mark clearance in the first quarter of 2008.
The company expects its DC-3 color ultrasound diagnostic imaging system to be launched in China and international markets upon receiving CE Mark clearance in the first quarter 2008. The DC-3 is designed to have wide applications in abdominal, OB/GYN, endovaginal, cardiac, small parts and pediatric markets. The product is suited for hospitals and clinics seeking replacement of black and white ultrasound systems.
The company expects its EX55 and EX65 compact anesthesia machines to receive CE Mark approval during the first quarter 2008.
The company will apply for FDA 510(K) clearance for its Beneview T5, T6, T8, PM-60, BS-200 chemistry analyzers and both its M5 and DC-3 diagnostic ultrasound systems. Mindray has to date received FDA 510(K) clearance for a total of 11 products, covering patient monitoring devices, diagnostic laboratory instruments and ultrasound imaging systems.
For more information: www.mindray.com


 July 19, 2024
July 19, 2024