ProxiScan
April 14, 2010 - For the first time, the U.S. Food and Drug Administration (FDA) gave 510(k) clearance to a gamma camera indicated for endocavity imaging.
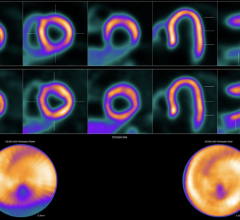
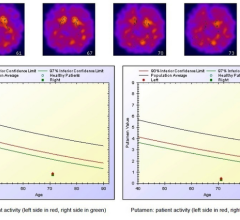
The technology is designed to address a widespread need for higher quality nuclear-medical images for prostate cancer diagnosis. ProxiScan is a small cadmium zinc telluride (CZT)-based compact gamma camera developed by Hybridyne in collaboration with scientists at Brookhaven National Laboratory.
The system can be used in imaging the distribution of radionuclides in the human body, using planar imaging techniques. The system may also be used intraoperatively, on pathological specimens and for endocavity applications if a protective sheath is used. ProxiScan is also capable of imaging radiopharmaceuticals distributed within anatomical regions of interest located close to the camera head.
For more information: www.hybridyneimagingtechnologies.com

 January 19, 2024
January 19, 2024