December 14, 2009 - The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for a lesion-localization system for molecular imaging biopsy guidance.
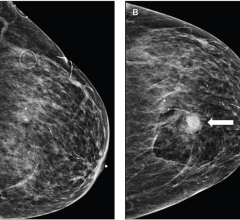
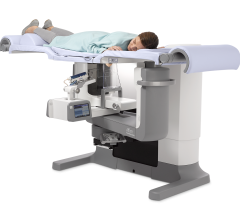
The system, GammaLMc, is designed to help doctors accurately locate breast lesions and enable gamma-guided biopsies. The GammaLMc system utilizes a CorreLocator paddle and a StereoView imaging collimator system, which is a technique similar to that used in stereotactic X-ray localization, and the GammaLMc software calculates the specific location of the suspect lesion. The compact design allows for breast biopsies with optimal patient comfort; and the entire system is small and portable, allowing physicians to perform molecular imaging guided biopsy procedures anywhere on site.
GammaLMc is a complementary technology to Dilon Diagnostic’s cornerstone product, the Dilon 6800 Gamma Camera. The device is particularly useful for patients that have findings on the Dilon system that are not revealed with other imaging modalities.
For more information: www.dilon.com


 September 14, 2023
September 14, 2023