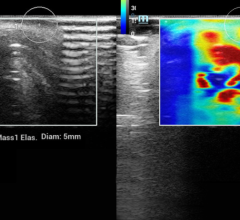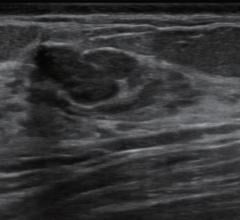
The suspicion that this ill-defined echopoor mass was malignant was supported by very high kPa values (over 200) in its periphery. The histology was a ductal carcinoma.
Palpation, amongst the oldest of clinical skills, was used in ancient Egypt and is described in the Ebers’ papyrus (about 1552 BC) to feel for hard lumps in the breast as an indicator of malignancy (Sakorafas, G H. 2001). Essentially this is what is done with elastography: A region of tissue is subjected to a compression force (called stress) and the degree to which it distorts (known as strain) is assessed. While in principle any imaging technique could be used, ultrasound has the advantages of good resolution in both space and time and of being safe and thus repeatable, so it has emerged as the dominant technique, while MR also has a role.
The stress can be applied in several different ways, but the most common is simply to compress the tissue with the transducer itself; only minimal compression is needed, moving the tissue by a few millimeters. Other external forces, such as mechanical vibrating devices and acoustic energy, have also been used in an attempt to make the technique less operator dependent. The simplest way to measure the strain is to track the movement of the tissue either by comparing single ultrasound lines (A-mode tracking) or by comparing the B-mode images before and after compression or continuously during repeated compression movements. In the most popular implementation, real time elastography, this is performed in two dimensions so that sideslip can be measured (as long as it remains within the scanned plane). The obvious extension to three dimensions has not yet been implemented as it is computationally too demanding.
Some ultrasound companies, such as Hitachi and Siemens, have introduced systems equipped with strain elastography, signaling the importance of this additional information in the diagnostic process. These are qualitative elastography methods. More recently, a unique and quantitative technology, Shear Wave Elastography, has emerged. Shear Wave Elastography uses the acoustic radiation force of the ultrasound wave to push the tissue, rather like an acoustic puff. This can be achieved at surprisingly low mechanical indices, well within the FDA-recommended upper limit of 1.9, and this has been implemented in the Aixplorer system, introduced by SuperSonic Imagine. An important benefit of generating the displacement acoustically is that it removes the user-dependence of strain elastography because no manual compression is applied to the tissue. Thus, it should be easier to learn and more reproducible.
This acoustic push is achieved by sending repeated focused pulses down the intended push line, each with a longer pulse length than is optimum for imaging; in fact, they are very similar to the pulses used for color Doppler and are generated with a conventional transducer. The push pulses follow closely upon each other such that the speed of travel of the resulting push is faster than the speed of sound in tissue; this supersonic push generates a sonic shock that amplifies the effect of the push beam. The to-and-fro particle motion that is generated triggers a conical shear wave that travels sideways away from the push line.
Shear waves differ in important ways from the more familiar longitudinal compression waves that constitute sound, both audible and ultrasound. They are analogous to the ripples on a pond’s surface: The particles move up and down while the wave travels horizontally, the movement being transmitted because of elastic forces between adjacent particles. Acoustic shear waves travel much more slowly than compression waves (1-10 m/sec compared with 1540 m/sec in tissue) and their velocity is proportional to the elasticity (Young’s modulus) of the tissue. They require an elastic medium to support them, so true fluids do not conduct them. The conducting particles only move up and down by a few microns, and this displacement cannot be detected by conventional ultrasound, as it only lasts for a few milliseconds in tissue, so a special imaging mode is required to demonstrate them.
The speed of the shear wave is measured by the Aixplorer system in a unique manner using ultra fast imaging in which a single unfocused (flat) pulse is transmitted instead of the conventional successive transmit-receive cycles required to form an image. Focusing is applied to the returning echoes. The acquisition speed needed to visualize a shear wave is 5,000 Hz, 200 times greater than available in conventional systems.
The resulting images of the shear wave’s velocity are displayed as a real time overlay on the simultaneously acquired B-mode, much as is conventional with color Doppler. Since only the speed of the shear wave is measured, the information on the tissue elasticity is quantitative and the color scale is calibrated in kilopascals; measurement tools give a numerical readout and can be used to compare the kPa of areas of interest within the image.
The advantages of shear wave elastography over strain elastography are its quantitative nature, its user-independence and its reproducibility, an exciting example of progress in ultrasound imaging.
These advantages are indeed emerging from the initial clinical experiences and will be tested formally in the ongoing multinational clinical trial.
Clinical Results
Developmental versions of the Aixplorer system have been tested over the last year and some preliminary results are available for the breast, in which the most experience has been gained. Clear patterns are emerging with cysts mostly showing as signal voids and fibroadenomas showing kPa values about the same as the surrounding breast tissue (around 20). Somewhat surprising but gratifying, is that several examples of fibrotic tissue, which often have very suspicious appearances on B-mode, have been found to have low kPa values, thus offering a potential way to avoid biopsy in these cases which often are scored as BI-RADS 3 or even 4.
Malignancies generally have high kPa values (usually over 100) but, unexpectedly based on their known histology and the finding from compression elastography, these high values are often apparent only in the periphery of the lesion and somewhat lower values are often found in their centers. In some cases, a necrotic centre is responsible, but this does not seem to apply to all cases and the suggestion that the stiff tumor somehow shields its centre remains to be explored. A part of this finding is that cancers show much greater heterogeneity of kPa values than benign lesions, and this was not a surprise.
The finding on compressional elastography that cancers appear larger on elastography than on B-mode, has been substantiated with the Shear Wave elastography of the Aixplorer. It is attributed to the stiffening of the tissues around the malignancy by infiltrating strands of tumor cells and by the fibrous reaction they elicit.
It was anticipated that some cancers would not appear to be stiff; colloid and lobular cancers are examples that are often impalpable and might fall into this category. In practice, in these preliminary results, several colloid cancers have in fact shown kPa readings in the malignant range, and so have not been false negatives. Invasive lobular carcinomas are rarer, but at least one case was a false negative, with low kPa values.
In situ ductal carcinomas (there is minimal experience with in situ lobular cancers to date) also generally appear with high kPa values and this could be important in guiding biopsies since these tumors are often invisible on B-mode.
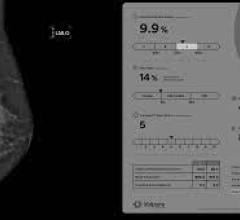
These early results encourage the suggestion that the addition of shear wave elastography to conventional B-mode and color Doppler grading may allow more lesions in the indeterminate BI-RADS 3 and 4 categories to be re-classified and thus improve patient management. For example, if a BI-RADS 3 lesion can be moved to 2 because it shows low kPa values, then follow-up or possibly a biopsy could be avoided. Similarly, the astonishing range of risk of malignancy of BI-RADS 4 lesions (7–94%) makes this classification a difficult one to use when it comes to advising patients on their optimum management. If a high kPa value is found, some may be moved up the BI-RADS 4 subscale or even into category 5, in whom a biopsy is always needed, while a low kPa value could allow a lesion to be reclassified as category 3 and encourage follow-up rather than a biopsy.
Future Prospects
From the breast study that is under way and aims to enter 2,300 patients, solid data on which to base recommendations for the use of shear wave elastography in patient management are expected. It should report by early 2010.
In principle, the way the Aixplorer generates the conical shear wave would lend itself to three-dimensional acquisitions and the provision of 3D shear kPa maps, a unique prospect amongst competing elastography techniques. It could also be used during biopsies; elastography is difficult when the probe needs to be moved to generate the elastogram in the conventional way.
The current implementation of Aixplorer with a high-resolution linear array, lends itself well to the study of other superficial structures. Limited studies on the thyroid, lymph nodes and musculoskeletal systems have been carried out, but it is too early even for provisional opinions to have emerged. Another intriguing possibility is that this technology might be capable of quantifying the elasticity of carotid plaque, which might predict the risk of stroke. Certainly, in this application, neither the immediately adjacent blood nor the movement of the artery wall impair the acquisition of elastography values (the blood is a signal void as it is a fluid and the kPa values are acquired so quickly by the flood imaging pulse that carotid wall movement does not interfere).
Plans are well advanced to develop a curved array suitable for abdominal use; obvious targets that will be of great interest are the liver and kidneys (especially transplants). In the longer run, intracavitary and endoscopic applications might also be of interest. The fast acquisition rate could lend itself to cardiac elastography, an intriguing possibility that would require a special transducer.
References:
Sakorafas, G H. Breast cancer surgery, historical evolution, current status and future perspectives. Acta Oncologia, Vol. 40: pp. 5–18, 2001


 July 24, 2024
July 24, 2024