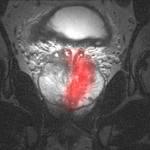
January 23, 2009 - Johnson Medtech, the medical products division of Johnson Electric, will participate in creating the world's first MRI-compatible image-guided tumor treatment device with Profound Medical Inc. (PMI).
This tissue coagulation device is expected to treat prostate cancer in a fraction of the time and cost of existing methods, based on extensive modeling, simulation and pre-clinical trials. Johnson Medtech's non-magnetic "Nanomotion" actuators enable the precision of motion and accuracy of treatment necessary for safely conducting the image-guided prostate cancer therapy within the strong magnetic field of the MRI.
PMI's device uses an MRI for imaging and a proprietary planar ultrasound applicator for treatment. The MRI precisely guides the probe that heats the cancerous tissue to effectively destroy the diseased area. In the past, the magnetic nature of electric motors and their metal components made it impossible for motorized medical devices to function within the MRI environment. To overcome this challenge, PMI selected Nanomotion's HR2-1-N-3 piezo ultrasonic non-magnetic motors to rotate the device's probe. When combined with the real-time non-invasive visibility into the human body provided by the MRI, the sophisticated low-speed Nanomotion actuators in PMI's device enable medical professionals to operate the probe at a microscopic scale to conduct this groundbreaking procedure.
For more information: www.johnsonmedtech.com and www.nanomotion.com


 December 04, 2025
December 04, 2025 









