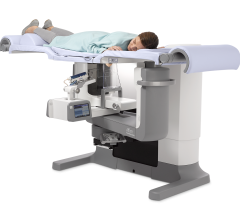
November 20, 2008 - Naviscan Inc. said the FDA has granted 510(k) clearance for its biopsy-guidance feature designed exclusively for use with its high-resolution organ-specific PET scanner, Positron Emission Mammography (PEM).
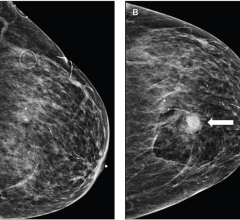
Stereo Navigator, the PEM-guided biopsy accessory, is indicated for the localization of lesions in female breasts, as identified on a PET image. This guidance system is designed to enable physicians to guide compatible interventional devices towards abnormalities visible on PET.
Naviscan will showcase the Stereo Navigator at RSNA 2008 next week in Chicago.
This Stereo Navigator biopsy feature reportedly represents the cutting edge of targeted molecular imaging in the breast. The accessory uses a stereotactic frame fixed between the scanner's paddles to guide the insertion of a compatible interventional device into the breast. Accurate targeting is possible due to the high-resolution 3D tomographic images acquired. Localization of the abnormality is verified using a PET-visible line source that is inserted into the needle track allowing the user to confirm trajectory and position. The accessory is compatible with the following breast biopsy systems: Mammotome from Ethicon Endo-Surgery Inc., ATEC from Hologic Inc., and EnCor from SenoRx Corp.


 September 14, 2023
September 14, 2023