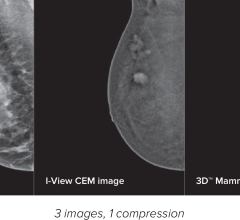
November 03, 2008 – The FDA has given 501(k) clearance to the Aurora 1.5Tesla Dedicated Breast MRI System for AuroraSPECTROSCOPY, Aurora Imaging Technology’s breast magnetic resonance (MR) spectroscopy package.
The availability of AuroraSPECTROSCOPY will provide Aurora Breast MRI the capability to perform in vivo breast MR spectroscopy (MRS) and MR spectroscopic imaging (MRSI). AuroraSPECTROSCOPY was developed to supplement Aurora Breast MRI by further improving the image specificity of this groundbreaking technology. The Aurora Breast MRI remains the only FDA-cleared dedicated breast MRI system specifically designed for the detection, diagnosis and management of breast disease alone.
Although the use of spectroscopy is in its very early stages for the indication of breast cancer, clinical studies have supported the potential of spectroscopy to improve image specificity in detecting malignant breast tissue.
For more information: www.auroramri.com


 July 24, 2024
July 24, 2024