Hologic's upgraded SecurView workstation recognizes breast imaging procedures based on procedure name or image content.
For the past two decades, breast cancer awareness has prompted women to take preventative measures that have helped them to beat the odds and live longer after initial diagnosis. But there is no complacency in the fight against breast cancer, and in just the last year, the industry has launched an arsenal of new diagnostic and therapeutic tools aimed specifically at defeating breast cancer.
To facilitate the transition from film to digital, Fuji blazed a new trail in 2006 with the long-anticipated commercial release of Fuji Computed Radiography for Mammography (FCRm). Etta Pisano, M.D., Kenan professor and director, UNC Biomedical Research Imaging Center, and one of the lead authors of the Digital Mammographic Imaging Screening Trial (DMIST), believes Fuji’s system will likely speed adoption of digital mammography in the U.S. “At UNC, we’ve just installed our first Fuji system and are planning for a phased roll-out of the technology at several of the University’s other breast imaging facilities,” noted Pisano.
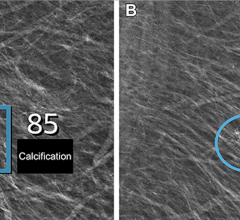
A new approach to digital mammography was also introduced with the REGIUS PureView Mammography System by Konica-Minolta Medical Imaging. The REGIUS is the only medical X-ray system that uses a Phased Contrast Imaging technique to enhance the edges of an image and improve contrast. By using a digital magnification technique, the system overcomes the blurriness typically found in magnified images. The resulting images enable the radiologists to readily visualize the edges of the mass, calcification and fibrils. The process also increases the resolution and sharpness of the image.
“The system does not lose resolution while gaining contrast,” said Robert A. Schmidt, M.D., professor of Radiology, The University of Chicago Hospitals. The compromise of digital mammography is that currently available systems provide lower resolution than film-screen mammography. Konica-Minolta has launched a pilot study to evaluate the Regius technology. Unique to this study is that it will be the first to compare the quality of image acquisition of different digital mammography and film-screen systems for diagnostic studies.
Vendors Pursue New Breast Imaging Devices
Ultrasound and MRI are gaining increasing acceptance with advanced breast imaging solutions. Aurora Imaging Technology Inc. introduced the first FDA- cleared MRI system designed specifically for breast imaging. The company developed and manufactured the Aurora 1.5T Dedicated Breast MRI System with Bilateral UltraRODEO as a diagnostic breast imaging tool for breast disease management.
Now its SpiralRODEO technique provides enhanced acquisition efficiency, reportedly three times greater than traditional 3DFT reconstruction, and resolution is dramatically increased along with a significant decrease in scan time for better dynamics. SpiralRODEO has applications in detection of cancer in higher risk women, characterization of indeterminate clinical or mammographically detected abnormalities, monitoring cancer response and residual disease after neoadjuvant chemotherapy, as well as evaluation of breast implant integrity with the silicone specific UltraRODEO sequence.
Clinicians can have complete medial and lateral access to the breast for unimpeded imaging and intervention with a new coupling of technology – Sentinelle’s Vanguard system, a dedicated breast imaging and intervention patient table, with GE’s Signa HD 1.5T MR scanners.
Holography offers a new perspective in breast imaging technology as Cedara Software and Advanced Imaging Technologies develop a breast ultrasound system that utilizes holography, or the diffractive properties of sound, to produce high spatial and contrast resolution images through an automated multiplanar paradigm. Imaging Diagnostic Systems will now provide CTLM laser breast imaging systems in to the U.S. market. Currently limited to investigational use in the U.S., CTLM is a patented breast imaging system that uses laser technology and patented algorithms to create 3-D images of the breast.
Promising New Studies
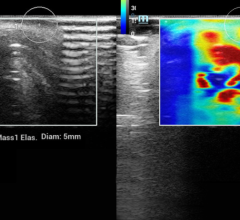
Breast imaging was a noticeable component of the 2006 RSNA meeting, and new strides are being made in not-so-new devices. Such is the case with a novel ultrasound technique that uses elasticity imaging to help radiologists accurately distinguish benign from malignant breast lesions. According to Richard Barr, M.D., Ph.D., professor of Radiology at Northeastern Ohio Universities College of Medicine and radiologist at Southwoods X-ray and MRI in Youngstown, OH, “elasticity imaging has been found to have high specificity.” He notes that if the results are reproduced in a large multicenter trial, the technique could significantly reduce the number of breast biopsies.
Siemens Medical Solutions plans to commercialize the free-hand elasticity imaging technique studied by Dr. Barr on its ACUSON Antares system, premium edition. The system will also be equipped with Advanced SieClear Spatial Compounding, a unique tissue stabilization technology, and Dynamic TCE (Tissue Contrast Enhancement) for B-mode imaging through speckle reduction for improved contrast resolution and image presentation.
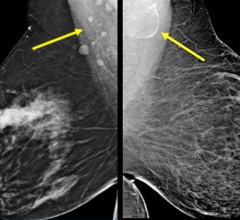
Preliminary results of a pilot study using the Cone Beam Breast Computed Tomography (CBBCT) scanner may equal or surpass mammography in detecting breast cancer. The technology, funded by the National Cancer Institute along with private investors, takes several pictures of the breast from various angles and creates a 3-D image. Although developed and patented by a radiologist at the University of Rochester, Koning Corp. holds the license for commercial development.
Dr. Joshua Kalowitz, chief of Breast Imaging at Maimonides Cancer Center in New York, tested the device only on healthy women and cautions that the device may not be able to pick up tiny abnormalities due to image resolution.
Electronic impedance for breast cancer screening is another novel approach. The works-in-progress Z-Tech Breast Cancer Detection System can detect breast cancer very early, based on clinical trials at over 20 hospitals and primary care facilities in the U.S., Canada and Europe. The device detects electrical changes associated with cancer; malignant lesions present an increase in the volume of fluid in the region and the cell membrane becomes electronically porous. Both of these changes, detected by the device, result in a decrease in electrical impedance in the cancerous area. A pivotal trial in 2007 will determine if the system meets the requirement for approval as a primary screening device for detecting breast cancer.
Brachytherapy Provides Treatment Options
A new approach to partial breast irradiation with brachytherapy may offer benefits over total breast irradiation in treating early stage cancer in some women. In a study by Lora D. Barke, D.O., assistant professor at Feinberg School of Medicine, Northwestern University and Northwestern Memorial Hospital, Chicago, IL, assessed the use of ultrasound to guide the placement of the balloon catheter before partial breast irradiation therapy with brachytherapy.
“Our research shows that immediate placement of the balloon catheter is unnecessary and may add to cost. Radiologists can wait until receiving the final pathology and then safely and efficiently insert the catheter with ultrasound guidance immediately before the patient begins brachytherapy,” noted Dr. Barke.
Accordingly, BioLucent’s new SAVI applicator offers a multicatheter, single-entry approach to accelerated partial breast irradiation after lumpectomy. The device combines the tissue-sparing dosimetry of interstitial brachytherapy with the single-entry ease of intracavitary, or balloon brachytherapy. By delivering a precise, targeted approach, more flexibility in treatment planning is possible.


 July 29, 2024
July 29, 2024