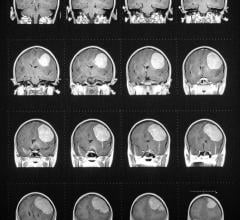
September 15, 2008 - Results from a safety study indicating that HX4, a new imaging biomarker that enables imaging hypoxic tumors, is safe for use in human positron emission tomography (PET) imaging studies, were presented on September 12, 2008, at the World Molecular Imaging Conference in Nice, France.
The biomarker was developed at Siemens Molecular Imaging Biomarker Research, and the study was executed in collaboration with Dr. Jian Q. (Michael) Yu and Fox Chase Cancer Center in Philadelphia. The study included initial human data regarding bio-distribution of the new agent, radiation dosimetry levels in normal volunteers and optimal patient imaging parameters with PET. Results of the study indicated that the compound was found to be stable for imaging at 145 minutes post injection, that it would safely clear the body through urinary elimination and that there were very low dose accumulations in major organs.
"Being able to image hypoxic tumors may significantly change the management of disease in cancer patients. The prognostic value of this level of information can effectively improve quality of life for oncology patients, offering them potential for personalized and possibly more effective treatment," said Hartmuth Kolb, vice president, Siemens Molecular Imaging Biomarker Research. "Siemens is committed to developing new methods to visualize disease processes with new imaging biomarkers in conjunction with our molecular imaging technology so that, ultimately, providers can detect and manage the treatment of disease much earlier."
Hypoxic cells are clinically problematic and tend to be less responsive to standard treatment regimens. A probe that measures hypoxia could prove quite a useful tool for oncologists. The development of an imaging biomarker that selectively identifies hypoxic tumor cells could help radiation oncologists tailor specific treatment options to most efficiently manage disease.
Siemens Molecular Imaging Biomarker Research facility in Los Angeles is dedicated solely to the discovery and development of new imaging biomarkers to spur the growth of in vivo molecular diagnostics. The facility houses scientists dedicated to the discovery of new imaging agents and their clinical development, with the goal of bringing several new agents to the market over the next five to 10 years. Research and development efforts conducted at the facility focus largely on oncology and neurology, and also include other areas, such as inflammation and microfluidics/nanotechnology research
This imaging biomarker is intended for exclusive worldwide distribution by PETNET Solutions, a fully owned Siemens subsidiary.
For more information: www.petnetsolutions.com and www.siemens.com/healthcare


 July 09, 2024
July 09, 2024