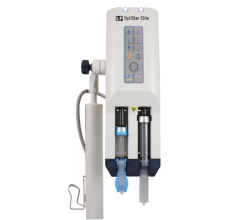
June 18, 2008 – MEDRAD gained FDA 510(k) clearance for a new Fluorodeoxyglucose (FDG) infusion system for positron emission tomography/computed tomography (PET/CT) imaging procedures, reportedly the first infusion system available in the U.S. to automate the FDG delivery process.
The new PET infusion system, Intego, reportedly enables the healthcare provider to easily administer FDG at any time throughout the day and enhances the clinician's ability to deliver FDG with precision, flexibility and safety.
The system automatically extracts a patient dose from a multidose vial and delivers it directly to the patient, virtually eliminating manual dose preparation and handling, and the corresponding radiation exposure to the technologist.
With the Intego System's dose-on-demand capability, the prescribed dose can be delivered when the patient and technologist are ready, letting technologists respond to schedule changes, patient delays, and add-on patients. The system has new features including real-time dose availability information, an integrated ionization chamber and an optional weight-based dose calculation, allowing the healthcare provider to more precisely customize each patient's dose.
Safety features include a tungsten multidose vial shield, a fully lead-lined mobile cart and an automated saline flush to remove residual FDG from the line after each infusion.
The company is working with FDG suppliers to provide FDG in multidose vials and vial shields compatible with the Intego System and recently announced a distribution and co-marketing agreement with the largest PET radiopharmacy network, PETNET Solutions, a fully owned subsidiary of Siemens Medical Solutions.
For more information: www.medrad.com


 July 09, 2024
July 09, 2024