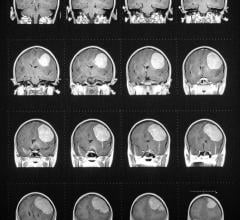
June 12, 2008 – The FDA designated for priority review, the New Drug Application (NDA) for GE’s AdreView, a molecular imaging agent for the detection of neuroendocrine tumors in pediatric and adult patients, also encouraging the company to establish an expanded access program that is designed to grant physicians limited access to a novel agent prior to FDA approval.
AdreView was submitted March 20, and accepted by the FDA May 20. A priority designation is intended for products or indications that address unmet medical needs. Under the Prescription Drug User Fee Act, the FDA's goal is to review and act on 90 percent of NDAs designated as priority review within six months of receipt.
The company began development of AdreView in 2004, and the agent was granted orphan-drug designation by FDA in December of 2006.
For more information: www.gehealthcare.com


 July 09, 2024
July 09, 2024