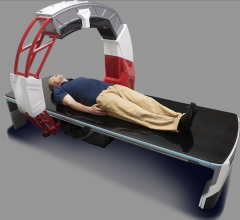
June 5, 2008 - NeuroLogica Corp. and its Korean distribution partner, Dong Kang Medical Systems, said the CereTom CT scanner has received clearance from the Korean Food and Drug Administration (KFDA) for medical capital device imaging.
The portable eight-slice CT scanner acquires 1.25-millimeter slices per rotation and is capable of performing NeCT, CT Perfusion, Xenon Perfusion and CT Angiography. According to the company, it is also optimal for use in the emergency room, intensive care unit and operating room because of its portability.
NeuroLogica also produces the CereTom OTOscan, a reportedly compact, lightweight, high-speed, wall powered eight-slice CT scanner optimized for full head and neck scanning up to C5.
With state-of-the-art image detail, the CereTom OTOscan is said to deliver effective, safe and flexible in-office CT imaging, according to NeuroLogica. The scanner delivers timely diagnosis and treatment planning in one appointment, reliving patient anxieties and saving time, the company said.
For more information: www.neurologica.com


 August 09, 2024
August 09, 2024