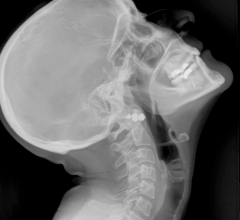
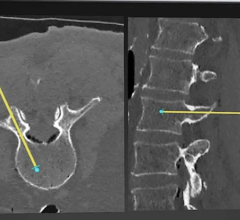
October 29, 2007 - Biospace med announced that it has received 510k clearance from the Food and Drug Administration (FDA) to market EOS, a new digital X-ray imager in the U.S. for 2D imaging use, reportedly allowing images to be obtained with a low dose of radiation and permitting long length digital imaging,
The system is said to allow full-body, uninterrupted digital imaging with a single scan. Information submitted to FDA in support of Biospace med’s marketing application demonstrated up to 10 times reduction in dose when compared with commercially available film systems, reportedly without compromising image quality.
System features include capturing a full or partial body image, large reduction in radiation dose and obtaining multi-planar (frontal and lateral) images simultaneously in an upright, weight bearing position
For more information: www.biospacemed.com


 July 18, 2024
July 18, 2024