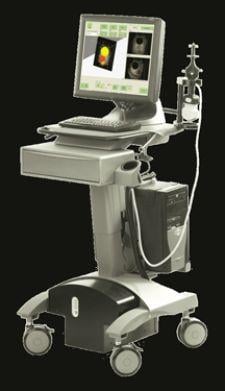
NOMOS Radiation Oncology, a division of North American Scientific, will bring to the table its BATCAM Multi-probe, a new IGRT solution that received FDA 510(k) approval on July 11, 2007.
The BATCAM Multi-probe integrates a new multi-probe capable ultrasound system into the company's core IGRT product, BATCAM. The new system features an updated abdominal ultrasound probe featuring color doppler ultrasound, enabling IGRT for liver and pancreas treatments in addition to prostate and gynecology.
Optional add-ons to the system include a bi-planar rectal probe for prostate volume studies and prostate brachytherapy and a wideband, linear probe for verification of Accelerated Partial Breast Irradiation (APBI) brachytherapy devices such as ClearPath.


 November 03, 2025
November 03, 2025 









