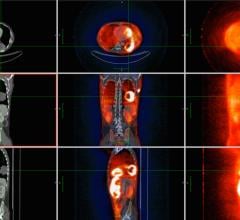
GE Healthcare has received FDA clearance for the next-generation volume PET/CT application, PET VCAR (Volume Computer-Assisted Reading). It was created to help clinicians diagnose, stage, treat and monitor tumors and other lesions in the body.
PET VCAR offers several workflow enhancements for both single and multi-exam review, including exam-to-exam automatic registration, tumor segmentation and quantification and multi-planar image review.
The FDA clearance includes a GE-patent Interactive Data Analysis (IDA) tool, which allows physicians to systematically track treatment over time and quantitatively interpret a patient’s response to therapy. The new IDA capability reportedly facilitates informed, objective treatment decision making by automating several previously manual processes and presenting data in an organized, user-configurable format.


 July 25, 2024
July 25, 2024