If you enjoy this content, please share it with a colleague
Riverain Technologies
RELATED CONTENT
Feb. 4, 2025 — Riverain Technologies recently announced it expanded across eight countries in 2024 and added nearly 50 ...
February 1, 2024 — Nearly 8,000 veterans are diagnosed with lung cancer each year. Now at-risk veterans will have access ...
September 6, 2023 — Riverain Technologies, the innovator of ClearRead solutions with Clear Visual Intelligence (CVI) ...
June 7, 2023 — Riverain Technologies, a medical device company revolutionizing chest imaging interpretation with Clear ...
December 3, 2020 — Riverain Technologies, a U.S.-based developer of artificial intelligence (AI)-powered software that ...
December 2, 2020 — Riverain Technologies will showcase its artificial intelligence solutions and enhancements at the now ...
September 17, 2018 — National outpatient physician radiology group SimonMed Imaging has selected Riverain Technologies’ ...
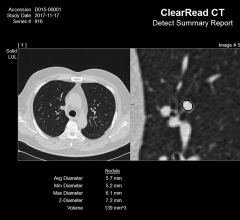
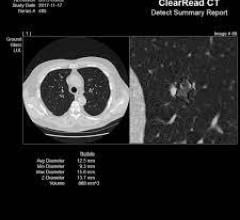
Riverain Technologies announced that the United States Patent and Trademark Office (USPTO) has awarded the company a broad patent for its artificial intelligence (AI) computed tomography (CT) technology. The technology in question forms a vessel suppressed, chest CT series using deep learning and synthetic data modeling. The company said the patent holds promise for improving the detection and diagnosis of lung cancer at an earlier stage while making clinicians more efficient, a first for computer-aided detection (CAD).
June 22, 2017 — Riverain Technologies announced that it is now processing data from nearly 50 computed tomography (CT) s ...
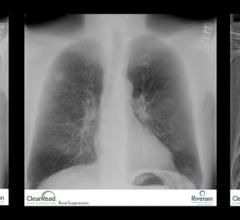
UCHealth (Colorado) has expanded their use of Riverain’s ClearRead Bone Suppression and ClearRead Confirm software to all five of their flagship hospitals. The expansion will ensure patients across the entire network benefit from the same tools designed to aid in more effective detection of lung disease, including lung cancer. Utilization of the ClearRead tools across UCHealth ensures patients and clinicians benefit from the technology independent of the imaging location within the network.


 February 04, 2025
February 04, 2025