If you enjoy this content, please share it with a colleague

Esaote North America
RELATED CONTENT
Introduced in the 1980s, magnetic resonance imaging (MRI) has become an integral part of any radiology department. The modality has most often been used for brain and spine scans, but new applications are expanding its capabilities in recent years. Vendors are also working to reduce both scan times and machine size for enhanced productivity.
This was a strong year for ultrasound technology, with several groundbreaking systems and technologies making waves in ...
Navigating coverage decisions can be overwhelming and confusing, especially for high-end imaging equipment, where ...
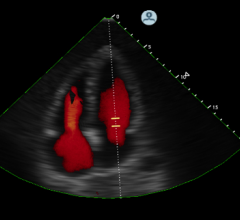
More than 50 companies and organizations will display their latest products and services at the American Society of Echocardiography’s (ASE) 26th Annual Scientific Sessions, June 12-16 at in Boston, Mass. ASE 2015 is the world’s premier meeting for cardiovascular ultrasound practitioners, and promises a wealth of cutting-edge education, research, and the latest vendor technology.
The latest advances in echocardiography were discussed at a special evening symposium at the 2015 American College of Cardiology (ACC) meeting, organized by Methodist Debakey Heart and Vascular Center.
At the 2015 European Congress of Radiology (ECR 2015), Esaote introduced Evolution’15 (EVO’15) as the latest upgrade in its dedicated magnetic resonance imaging (MRI) Evolution program. EVO’15 combines software updates and new hardware features to provide high image quality and increases productivity by almost 50 percent.
The future of ultrasound looks auspicious, according to Jonathan M. Rubin, M.D., Ph.D., director of the division of ultrasound in the department of radiology at the University of Michigan in Ann Arbor, who presented on “The Future of Ultrasound” at the 2014 Radiological Society of North America (RSNA) conference in Chicago this past December. His lecture highlighted emerging techniques for volume blood flow and shear wave imaging.
The U.S. ultrasound market reached an all-time high of $1.44 billion in 2013 — a growth of almost 3 percent over 2012, according to Klein Biomedical Consultants’ “The Medical Diagnostic Ultrasound Market in the USA: Challenges & Opportunities in the New Millennium” 2013 report. Areas that contributed to market growth included musculoskeletal ultrasound and point-of-care ultrasound, which saw double-digit growth in 2013. “In spite of the uncertainties caused by the Affordable Care Act, continued declines in reimbursement and slow economic growth, we saw an uptick in ultrasound purchases,” said Harvey Klein, Ph.D., market analyst and author of the report.
October 3, 2014 — Esaote North America announced that its new MyLab Gamma ultrasound system has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is now available for sale in the United States.
September 8, 2014 — Esaote North America announced its new MyLab Six ultrasound system has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is now available for sale in the United States.

 April 05, 2016
April 05, 2016