If you enjoy this content, please share it with a colleague
RELATED CONTENT
January 29, 2020 — EOS Imaging announced the first installation of its new EOSedge system in North America at CHU Sainte ...
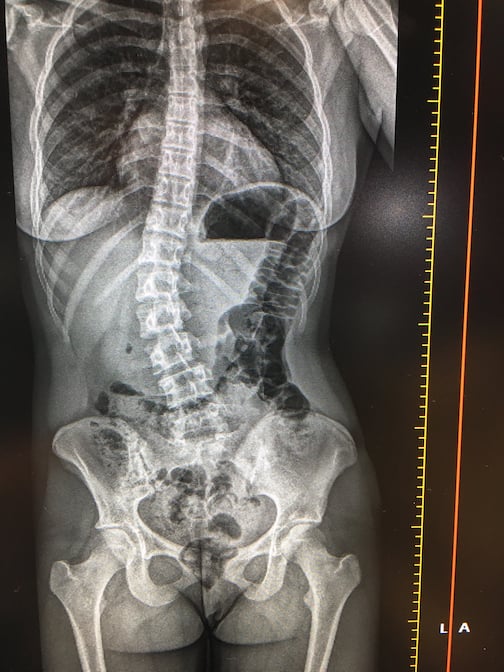 This is a demo of the EOS orthopedic X-ray imaging system at the recent 2019 Radiological Society of North America (RSNA ...
This is a demo of the EOS orthopedic X-ray imaging system at the recent 2019 Radiological Society of North America (RSNA ...
October 29, 2019 — MemorialCare Miller Children's & Women's Hospital Long Beach is now the only children's hospital from ...
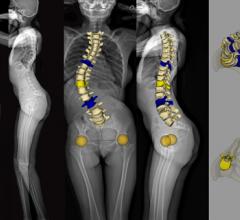
October 24, 2019 — EOS imaging announced the first patient cases performed with its hipEOS 3.0 surgical planning ...
EOS imaging recently announced the first installation of an EOS system in Mexico, the largest Central American market, at Shriners Hospitals for Children – Mexico, located in Mexico City.
EOS imaging announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its hipEOS 3.0 surgical planning software.
EOS imaging hosted a symposium entitled “How 3-D Weight-Bearing Planning from EOS Images Contributes to Improving THA Outcomes” during the American Association of Hip and Knee Surgeons (AAHKS) Annual Meeting, Nov. 2-5 in Dalls. The AAHKS Annual Meeting addresses a broad array of scientific topics such as implant design, outcomes, surgical techniques and complications of primary and revision total joint arthroplasty (TJA) for hip and knee surgeons.
EOS imaging announced the sale of the 10th EOS system to the Shriners Hospitals for Children Network in the United States, to be installed in Los Angeles.
EOS imaging, a in 2D/3D orthopedic medical imaging, announced today that the U.S. Food and Drug Administration (FDA) has approved spineEOS, an online 3D planning software for spine surgery based on EOS stereo-radiographic 2D/3D imaging.
EOS imaging announced that it has received CE Mark for spineEOS, its online 3-D planning software for spine surgery based on EOS bi-planar imaging. spineEOS is the third “EOSapp” software planning solution introduced by the company, joining its hipEOS and kneeEOS products.


 January 29, 2020
January 29, 2020