If you enjoy this content, please share it with a colleague
Bracco Diagnostics
RELATED CONTENT
Bracco Imaging announced that the first echocardiography imaging procedure with Lumason was performed in May at Morristown Medical Center, part of the Atlantic Health System, in New Jersey. This is the first administration after the development of the agent (sulfur hexafluoride lipid-type A microspheres) in the United States and approval by the U.S. Food and Drug Administration. The announcement was made through Bracco Imaging’s affiliate Bracco Diagnostics Inc.
Bracco Imaging, through its business unit Bracco Injeneering SA, announced the availability of its EmpowerCTA+, an advanced engineering contrast injection system.
Cardiac imaging accounts for about one-third of the source of X-ray radiation dose for all medical imaging. Expanding use of computed tomography (CT) for cardiac evaluations, use of nuclear imaging for myocardial perfusion exams and more complex transcatheter procedures in the cath lab have all increased patient exposure in recent years.
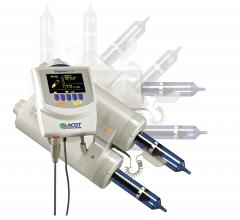
There have been several recent advances with contrast media injector technology, including cutting costs by reducing contrast waste and automated data collection for the dose a patient receives. Automated injector systems not only precisely control the amount of contrast used; vendors have moved into the software/IT arena by offering personalized doses for patients using information pulled from an electronic medical record (EMR) or picture archiving and communication system (PACS). Contrast dose recording software also offers new possibilities for radiology departments looking to streamline and document their contrast media usage. Some vendors have now integrated X-ray radiation dose recording capabilities as well.
Bracco Diagnostics Inc. announced the availability of its Isovue (iopamidol injection) Imaging Bulk Package (IBP), a specific combination multi-patient, multi-dose compliant contrast medium approved by the U.S. Food and Drug Administration (FDA) for point-of-care use in the computed tomography (CT) suite.
The U.S. Food and Drug Administration (FDA) approved Lumason (sulfur hexafluoride lipid microsphere) for patients whose ultrasound image of the heart (echocardiograms) are hard to see with ultrasound waves.
The use of contrast media in many imaging modalities continues to evolve as physicians seek to improve dose management practices and vendors churn out automated injectors that can precisely control the amount of contrast and personalize doses for patients using information pulled from an electronic medical record (EMR) or picture archiving and communication system (PACS). The features of today’s contrast media injectors include syringeless options and dose recording software, offering new possibilities for radiology departments looking to streamline and document their contrast media usage.
The average age of installed MRI scanners in the United States has increased from 8.7 years in 2010 to 11.4 years in 2013, according to a new market research report by IMV Medical Information Division.
DAIC Editor Dave Fornell highlights some of the biggest trends and most innovative technology discussed during the ...
Bracco will feature the latest in contrast delivery systems at RSNA. Consistent with the theme of this year’s meeting, “Patients First,” these systems offer cutting-edge technology to impact patient care, workflow and operational efficiencies.


 June 04, 2015
June 04, 2015