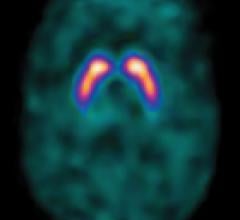
January 25, 2011 — Royal Philips Electronics has announced CE marking for the industry’s first commercially available whole-body positron emission tomography/magnetic resonance (PET/MR) imaging system, the Ingenuity TF PET/MR. The system is pending 510(k) and not available for sale in the U.S.
January 24, 2011 – Lantheus Medical Imaging has entered into a contract with Novation for Ablavar (gadofosveset trisodium) and Definity Vial for Injectable Suspension (Perflutren Lipid Microsphere). The agreement will provide increased access to the diagnostic imaging agents for healthcare providers served by Novation.
January 24, 2011 – A medical picture archiving and communication system (PACS) is now compatible with Apple’s smart phones, portable tablets and other devices. The system, by New Mexico Software, is available on the Apple App Store. It is part of the company’s effort to engineer mobile medical applications for Apple’s iOS and Google’s Android “Honeycomb” operating systems.
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
January 24, 2011 – New specimen X-ray software works with an algorithm to synthesize the ability of the human eye to locate information and analyze structures within the image. The result is digital mammography quality images with a digital radiography (DR) system.
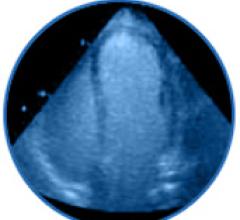
Contrast-enhanced ultrasound (CEUS) can "dramatically improve" physicians' ability to detect heart disease and stratify future risk, said Thomas Porter, M.D., at the 16th European Symposium on Ultrasound Contrast Imaging in Rotterdam.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
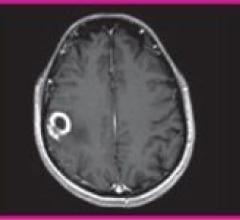
January 24, 2011 – A U.S. Food and Drug Administration’s (FDA) panel voted unanimously last week to recommend the clearance of a magnetic resonance imaging (MRI) contrast agent to detect and visualize areas with disrupted blood brain barrier and/or abnormal vascularity of the central nervous system (CNS).
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
January 21, 2011 – The U.S. Food and Drug Administration (FDA) this week unveiled a plan containing 25 actions it intends to implement during 2011 to improve its 510(k) review of medical technology, the most common path to market for medical devices.
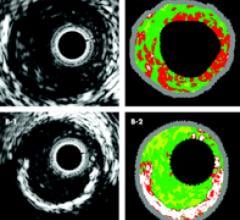
January 21, 2011 – Intravascular ultrasound (IVUS) enabled physicians to more accurately assess the risk of individual blockages than the use of the current standard of angiographic imaging alone, according to a new study.
January 21, 2011 — The U.S.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
The Society for Cardiovascular Angiography and Interventions (SCAI) this week published guiding principles and best practices for the development of radiation safety programs in cardiac catheterization laboratories.
January 20, 2011 – Telemis, the specialist in medical imaging solutions, has further consolidated its position in Italy with the acquisition of Micromedica Ltd, one of the country’s leading picture archiving and communication system (PACS) vendors.
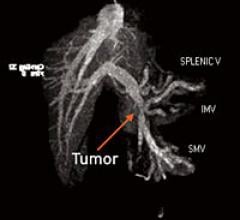
January 20, 2011 – Researchers say radiofrequency fields can successfully treat pancreatic tumors, which kill more than 95 percent of diagnosed patients. Kanzius Cancer Research Foundation (KCRF) announced research conducted in the Kanzius/Curley Lab at The University of Texas M.D.
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
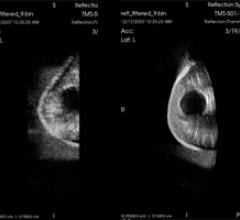
January 19, 2011 – TechniScan, a medical device company engaged in the development and commercialization of an automated 3-D breast ultrasound imaging system, said this week it signed a product development agreement with Womens3D Inc.
January 19, 2011 – GE Healthcare and Veran announced a strategic supplier agreement this week in which GE became the exclusive distributor and reseller of Veran’s ig4 fusion imaging angiography navigation system in the United States.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
January 19, 2011 – The U.S. Food and Drug Administration (FDA) cleared a radiopharmaceutical agent intended for use with single photon emission computed tomography (SPECT) imaging, for the detection of dopamine transporters (DaT) in the brains of adult patients with suspected Parkinsonian syndromes (PS).(1)
The FCR Go 2 is an upgraded version of the FCR Go portable digital X-ray machine, with a number of new features. It has an enhanced generator, full-size workstation and improved drive performance, all designed to provide high-quality images and ease-of-use to technologists conducting imaging exams outside the radiology department.
January 17, 2011 – Six winners have been selected to receive grants for their programs focused on patient care and safety in pediatric and adult imaging. They are: Northeast Georgia Medical Center, Gainesville, Ga.; Children’s Hospital and Medical Center, Omaha, Neb.; St.
This unique solution for collaborative diagnostic work improves decision-making efficiency in acute care, allowing physicians to efficiently set the care strategy in multi-trauma cases. It also serves as a platform for medical education, clinical conferences and virtual autopsies.
January 14, 2011 – Five-year outcomes on low-risk prostate cancer patients who were treated with a robotic radiosurgery system have been published in the January 10 issue of Radiation Oncology. The patients were treated with Accuray’s CyberKnife Robotic Radiosurgery System.
InSite One offers InDex vendor-neutral archive solutions for medical imaging and associated data. The imaging electronic health record (EHR) allows not only the physician, but also the patient to share medical data.

 January 25, 2011
January 25, 2011