The U.S. Food and Drug Administration (FDA) published a final guidance document entitled “Pediatric Information for X-ray Imaging Device Premarket Notifications,” encouraging manufacturers to consider child safety when designing X-ray imaging devices. In the guidance, which applies to new devices and modifications requiring the submission of a new 510(k), the FDA recommends manufacturers design these devices with protocols and instructions that address use on pediatric patients. It encourages the inclusion of pediatric indications and provides recommendations for labeling and instructions for use for X-ray imaging devices likely to be used on children.
Philips recently announced agreements with 3D Systems and Stratasys, two global leaders in the 3-D printing industry, to help progress patient care and improve the clinician experience. Advanced 3-D modeling provides radiologists with additional views to help strengthen anatomical knowledge which could enhance clinical impact in reviewing complex, multi-disciplinary cases.
ITN and DAIC Editor Dave Fornell takes a tour of some of the most interesting new medical imaging technologies on the ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
Nancy M. Cappello, Ph.D., executive director and founder, Are You Dense Inc. and Are You Dense Advocacy. Read the ...
When it comes to pediatric patients, having all the most advanced technology in the world counts for next to nothing if ...

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
A post-game roundup by ITN Contributing Editor Greg Freiherr and ITN Editor Dave Fornell on the key trend of artificial ...
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Emanuel Kanal, M.D., director of MRI services and professor of radiology and neuroradiology at the University of ...
RayStation 7, the latest release of RaySearch’s radiation therapy treatment planning system, adds new functionality and a wide range of improvements. Significant additions include support for Hyperscan technology from Mevion Medical Systems, and integration with RayCare, the next-generation oncology information system (OIS) shortly to be launched by RaySearch.
December 19, 2017 — Humanetics Corp. presented data at the annual meetings of the American Society for Radiation ...
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
December 19, 2017 — IBA (Ion Beam Applications SA) announced that Miami Cancer Institute treated its first patients ...
December 19, 2017 — Toshiba Medical, a Canon Group company, introduced the Galan RT Solutions suite of radiation therapy ...
iCAD Inc. announced the results of a landmark study that showed the benefits of adjunct intraoperative radiation therapy (IORT) compared to external beam radiation therapy (EBRT) in the treatment of early-stage breast cancer. The analysis demonstrated that IORT could result in direct cost savings for the U.S. healthcare system of more than $630 million over the lifetime of patients diagnosed annually with early-stage breast cancer, as well as significantly benefit patient health by minimizing radiation exposure and offering a better quality of life. The results of the study, which were originally published in the peer-reviewed Cost Effectiveness and Resource Allocation, determined IORT to be the preferred method of treatment.
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
The U.S. Food and Drug Administration (FDA) announced in a new drug safety communication is this week that it is ...
Over the past couple years covering the news about gadolinium-based contrast agent (GBCA) retention in the brain, skin ...

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
A post-game roundup by ITN Contributing Editor Greg Freiherr and ITN Editor Dave Fornell on the trends and new tech seen ...
At the 2017 Radiological Society of North America (RSNA) Annual Meeting, GE Healthcare and NVIDIA announced a series of imaging equipment advances powered by NVIDIA’s artificial intelligence (AI) computing platform. The announcements included the new Revolution Frontier computed tomography (CT) system, advancements to the Vivid E95 4-D Ultrasound and development of GE Healthcare’s Applied Intelligence analytics platform.
Optellum, a high-tech startup in lung cancer and machine learning, and Mirada Medical recently announced a partnership to accelerate the deployment of deep learning technology to market. This collaboration combines the deep technology and clinical expertise of Optellum with the software platforms and rapid development know-how of Mirada. They have partnered to address what they call a huge and growing problem in lung cancer diagnosis: the management of patients with indeterminate pulmonary nodules.
December 14, 2017 — iCAD Inc. recently announced that customer adoption of its PowerLook Tomo Detection is gaining ...
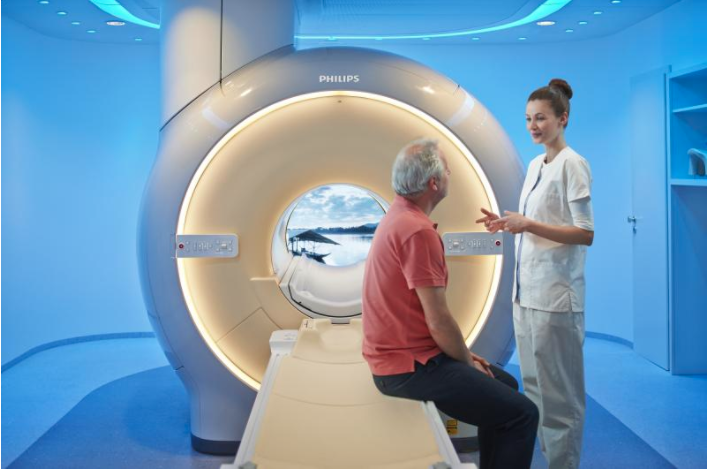
Philips showcased the company’s latest magnetic resonance (MR) imaging solutions at the Radiological Society of North America’s 2017 annual meeting, Nov. 26-Dec. 1 in Chicago. This includes their newest MR system, MR Prodiva 1.5T (which is still U.S. Food and Drug Administration [FDA] 510(k) pending) that provides enhanced clinical performance, workflow and patient experience. Philips also unveiled new solutions to drastically reduce exam times and elevate neuro-oncology.
Siemens Healthineers introduced four new computed tomography (CT) scanners across two platforms at the 2017 Radiological Society of North America (RSNA) Annual Meeting, Nov. 26-Dec. 1 in Chicago. The two new scanners in the Somatom go. Platform — Somatom go.All and Somatom go.Top — expand the mobile workflow into advanced fields such as cardiology and CT-guided intervention. With its new high-end systems for single- and dual-source imaging — Somatom Edge Plus and Somatom Force — Siemens Healthineers is introducing the new FAST (Fully Assisting Scanner Technologies) Integrated Workflow with the new FAST 3-D Camera.

 December 21, 2017
December 21, 2017