Incidental findings often are not afforded the weight of primary ones. But not following up on these incidentals can ...
Blue Earth Diagnostics, a molecular imaging diagnostics company, announced that a definitive agreement has been signed ...
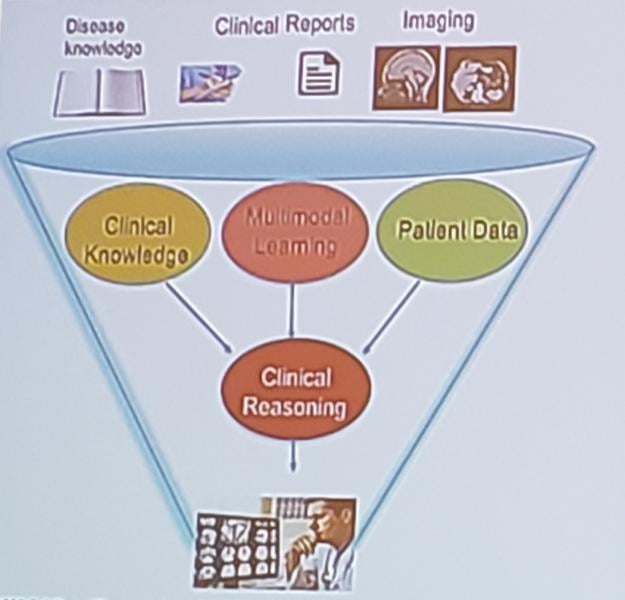
Radiology reports may contain information essential to figuring out a patient’s condition. But this information is often ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
The U.S. Food and Drug Administration (FDA) issued the final guidance: Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. This final guidance provides detailed recommendations for manufacturers seeking marketing clearance of diagnostic ultrasound systems and transducers.
Ambra Health announced an integration with Box to enable the sharing of medical imaging directly from within Box's cloud content management platform.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
ImageGrid Mini is a feature-rich, reliable and cost-effective image management and workflow optimization solution ...
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Kevin Seals, M.D., University of California San Francisco (UCSF) Health, interventional radiology fellow, is working on ...
Konica Minolta Healthcare Americas, Inc. and the Emory Healthcare Innovation Hub recently formed a strategic partnership ...
At the Society for Imaging and Informatics in Medicine (SIIM) Annual Meeting in Aurora, Colo., Nuance Communications ...
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Nina Kottler, M.D., vice president of clinical operations, Radiology Partners, explains how the company developed its ...
Barco’s new remote radiology reading solution ensures dependable imaging when radiologists are working outside the ...
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
Use of 3-D mammography, an advanced form of breast cancer screening, has risen rapidly in recent years, according to ...
Way too often the technologies we thought would be wonderful turn out to be the bane of our existences. When that ...

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
Siemens Healthineers and Mentice AB announced the collaboration to fully integrate Mentice’s VIST Virtual Patient into the Artis icono angiography system from Siemens Healthineers. The VIST Virtual Patient thus becomes a fully integrated simulation solution for the angio suite, according to the companies. The global partnership between the two companies will allow interventional radiologists, neuroradiologists, and cardiologists to perform vascular and cardiac interventions on a virtual patient inside the angio-suite.
Artificial intelligence (AI) imaging solution form ClariPi Inc. has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its AI-based computed tomography (CT) denoising technology, ClariCT.AI.
Medical imaging is ripe for the picking. Here’s why. Imaging and information systems are becoming ever more connected ...
Lawrence Tanenbaum, M.D., Radnet vice president and chief technology officer, discusses some of the artificial ...
DOSIsoft announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market Planet Onco Dose software for its oncology and yttrium-90 (Y-90) microsphere SIRT 3-D dosimetry components.
Advanced visualization and artificial intelligence (AI) technology provider TeraRecon has successfully completed a U.S. Food and Drug Administration (FDA) regulatory review of its Northstar AI Results Explorer. TeraRecon said the technology and the determination are both firsts-of-kind in the medical imaging industry. Northstar is designed to work alongside the company’s EnvoyAI interoperability platform, which includes FDA-cleared third-party content listed on its EnvoyAI Exchange marketplace.


 June 28, 2019
June 28, 2019