The purpose of this exploratory video roundtable is to examine the state of breast biopsies today and to communicate the current challenges and inefficiencies affecting personnel that perform these important procedures. The video roundtable posed key questions to Key Opinion Leaders from the Cleveland Clinic on obstacles they face when performing stereotactic-guided breast biopsies, and why it is necessary to consider the ramifications. The roundtable discussion was hosted by Imaging Technology News and was funded through a grant from Hologic Inc.
TeraRecon, together with full-color 3-D printing cloud provider WhiteClouds, announced new technological and workflow process to print highly detailed models directly from the TeraRecon software. The new 3D Print Packs make online, cloud-based 3-D reconstruction and printing simple, fast and affordable.
November 15, 2016 — Spellman High Voltage Electronics Corp. announced their DC high-voltage power supplies, X-ray ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
Sorna Corp. announced that it has received certification from British Standards Institution Group America Inc. that Sorna complies with the requirements of the internationally recognized ISO 13485:2003 standard. The standard is applied for the Quality Management Systems of organizations involved in the design, development, testing, manufacture and distribution of medical devices.
Clarius Mobile Health has introduced a new ultrasound scanner designed for veterinarians who treat household pets. The Clarius C7 Wireless Ultrasound Scanner pairs with iOS and Android devices to enable quick scans.

SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
NEC Display Solutions of America recently announced its decision to redirect development resources away from future medical diagnostic displays and instead to focus on its extensive healthcare display portfolio. With the growing need for displays in the healthcare segment, NEC said it is ideally placed to meet the needs of the market by offering a wide range of display solutions, such as healthcare clinical review displays, operating theatre displays, and healthcare information and signage solutions.
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
Lexmark International recently announced the availability of new solutions that further enable all patient content to be accessed and managed to improve patient-centered care.
HealthMyne will be unveiling and providing demonstrations of their new Quantitative Imaging Decision Support (QIDS) commercial platform at the 2016 annual meeting of the Radiological Society of North America (RSNA), Nov. 27-Dec. 1 in Chicago.
Medic Vision Imaging Solutions Ltd. announced that its U.S. Food and Drug Administration (FDA)-cleared SafeCT-29 will be at showcased at the upcoming Radiological Society of North America (RSNA) annual meeting, Nov. 27-Dec. 1 in Chicago.
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
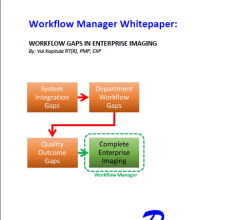
Regional Health Systems are experiencing an unprecedented consolidation as they try to establish an Enterprise Imaging (EI) strategy to take advantage of their newfound scale in their local markets. Development of an EI strategy has proven to be very challenging due to segregated informatics systems and isolated imaging silos and the reality of incorporating the next acquisition hospital or imaging center around the corner. Workflow Manager/Engine (WFM) technology, whether procured as a single application or already available with an implemented platform, allows healthcare providers to leverage existing imaging technology and establish early stages of an EI environment without major systems replacements.
British medical device maker BTG PLC and its subsidiary, Biocompatibles Inc., have agreed to pay $36 million to the U.S. government to resolve federal and state False Claims Act (FCA) allegations resulting from the off-label promotion of LC Bead, a medical device approved for use only for embolization of hypervascular tumors. $25 million of the settlement is to resolve the civil qui tam action, which alleged fraud, and $11 million of the settlement is a criminal fine against Biocompatibles.
November 11, 2016 — A European health consortium is developing a set of low-radiation, low-cost, flat panel X-ray ...
Did you know that approximately one-third of all the data in world is created by the healthcare industry and that ...
November 11, 2016 — Siemens Healthineers announced that University of Iowa Stead Family Children’s Hospital in Iowa City ...
Barco recently announced that Nexxis, its IP-based OR imaging management platform, is now operating in over 1,000 operating rooms worldwide.

SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
lifeIMAGE announced in mid-October the immediate availability of lifeIMAGE 5.0, the latest upgrade to its enterprise-grade electronic medical image sharing platform that connects thousands of hospitals across the country.
Siemens announced it intends to further develop its healthcare business, Siemens Healthineers, and give it even greater flexibility in implementing its growth plans. To this end, the company is planning to publicly list its healthcare business.
Carestream is accepting orders from healthcare providers across the globe and announced plans to begin shipping its OnSight 3D Extremity System in December.
Leveraging RESTful web services to unify communications between various health IT platforms the Unifier solves a wide ...
Telemis has launched the next generation of its picture archiving and communication system (PACS) software. Version 4.8 of the Telemis-Medical archiving software and image transmission platform features improved diagnostic mammography and comparison functionality alongside powerful visualization capabilities for users of mobile devices. The new version also further simplifies sharing of medical imaging data between medical practitioners and within and between medical facilities.
Augmenix Inc. announced that the American Medical Association (AMA) has granted a Category I, Current Procedural Terminology (CPT) code specifically for periprostatic implantation of biodegradable material. AMA granted the code with the support of the American Society for Radiation Oncology (ASTRO) and the American Urological Association (AUA).


 November 16, 2016
November 16, 2016